2021 review of CAR-T
~ a summary of the science and commercial landscape for this intriguing cell therapy
author: Kevin Curran PhD
updated: 8-4-2021
CAR-T is a form of immunotherapy.
Immunotherapy is a broad term for all disease treatments that work by either activating or suppressing the patient’s own immune system.
When an immunotherapy approach is used to attack cancer cells, we call this immuno-oncology.
On it’s own, our immune system does have the capacity to attack and kill cancer cells without medical intervention. But, the truth is, our immune system is only good at killing cancer cells, it’s not great. Cancer cells have already demonstrated they can outsmart our immune system and evade capture.
In response, CAR-T companies are pursuing a strategy that super-charges our own immune cells so that our T-cells gain a competitive advantage over cancer cells.
This strategy involves removing a patient’s blood then engineering T-cells to aggressively hunt down and kill cancer cells. In this sense, CAR-T is a living drug. The patient’s own immune cells are genetically modified to become the central component of this therapeutic product.
In this article, I review the CAR-T process in detail and discuss it’s effectiveness and safety issues. At the end of the article, I weigh in on future directions for CAR-T. I also review allogeneic CAR-T companies.
I’ll be updating this page every 6 months.
Click on a topic below or scroll down and read entire article.
Please share this article ↓↓
CAR-T is a great acronym for a very wordy treatment name.
The CAR-T acronym stands for Chimeric Antigen Receptor T-cell therapy.
As of 2021, Novartis, Kite/Gilead and BMS offer FDA approved CAR-T treatments for chemotherapy resistant lymphoma. Certain large cancer centers also manufacture their own CAR-T cells and offer patients this service in the context of clinical trials.
The image below illustrates key steps in CAR-T.
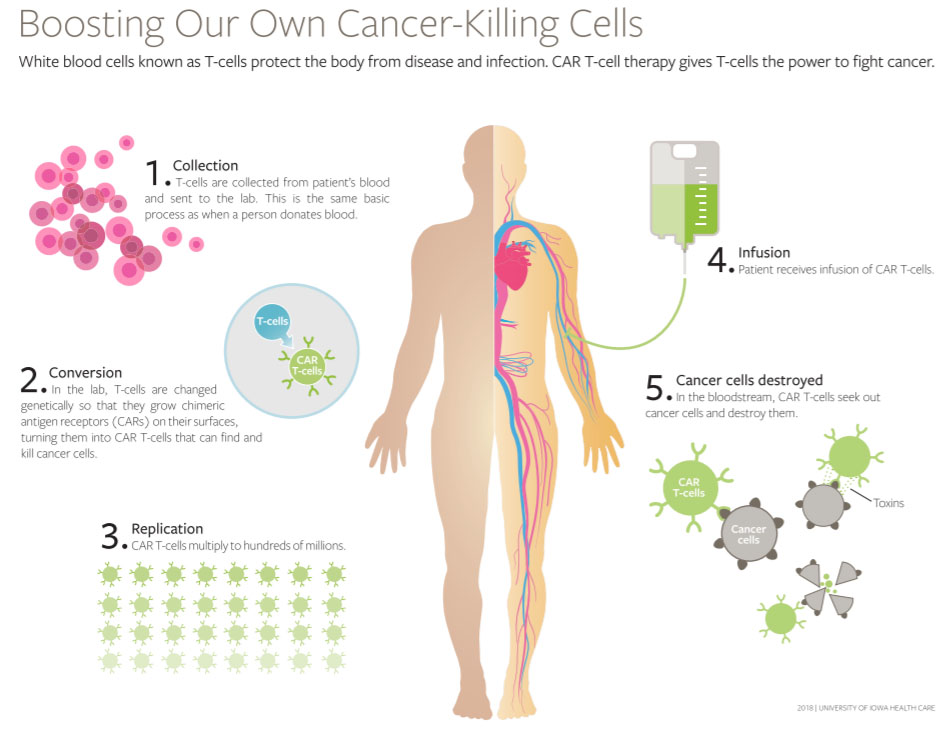
Image is copyright with the Univ. of Iowa and used with permission.
FDA approved CAR-T products
In late 2017, the FDA approved the first CAR-T therapy called Yescarta (axicabtagene ciloleucel). This treatment is approved for patients with either relapsed or refractory forms of non-Hodgkin lymphoma (NHL). The most common form of NHL is called diffuse large B-cell lymphoma, or DLBCL. A CAR-T company named Kite developed this product. Gilead bought Kite for 11.9 billion prior to FDA approval.
In 2018, the FDA approved a second CAR-T, Kymriah (tisagenlecleucel), for adults with a similar form of relapsed or refractory types of non-Hodgkin lymphoma. Kyrmiah was also approved for pediatric B-cell acute lymphoblastic leukemia (ALL). Kymriah is a Novartis product.
In March 2021, Breyanzi was also approved for DLBCL lymphoma and certain forms of follicular lymphoma. Breyanzi was developed by Juno Therapeutics. Juno was bought out by Celgene and then Celgene was bought out by Bristol Meyers. BMS is now marketing the drug.
Breyanzi, Yescarta and Kymriah are all slightly different versions of a CD19 targeting CAR-T.
The majority of lymphoma patients (approx. 80%) that are being prescribed Yescarta, Breyanzi and Kymriah are 3rd line DLBCL patients. 3rd line DLBCL patients do not have many treatment options, their cancer does not respond to chemotherapy.
In 2020, Kite also received FDA approval for Tecartus. This CAR-T is very similar to Yescarta but is indicated for mantle cell lymphoma patients.
In March 2021, BMS/Bluebird Bio was awarded approval for Abecma. Abecma is the first CAR-T to reach commercial status for multiple myeloma patients.
CAR-T process in more detail
- Cancer patient visits a hospital that is certified to deliver CAR-T. Clinicians draw blood from the patient. Patient is then free to go home.
- Patient’s blood is shipped on a plane to the manufacturing site of a CAR-T company.
- CAR-T company manufactures the T cell component of patient’s blood. Manufacturing process generally takes 2-3 weeks before the company ships manufactured cells back to the patient. Isolating T-cells from blood is called apheresis. Once isolated, gene therapy is administered to T-cells in an ex vivo context. A lentivirus or retrovirus is used to insert a CAR gene into the genome of isolated T cells. T cell population is expanded to reach desired cell count. At this point, the patient’s T cells are considered manufactured product.
- Manufactured CAR-T cells are shipped back to patient’s health care provider.
- Patient is notified their CAR-T cells have arrived at the hospital. The patient may receive a chemotherapy treatment (Flu/Cy) prior to the infusion of manufactured T cells. This chemo protocol is called lymphodepletion.
- Patient checks into the hospital on an inpatient basis.
- Patient receives an infusion of CAR-T cells.
- Patient remains inpatient while clinical symptoms are monitored by CAR-T trained physicians and nurses. Patient is observed for signs of cytokine release syndrome (CRS) and neurotoxicity.
- Once clinical conditions have stabilized, patient can move to outpatient status. Outpatient status may require that the patient stay within 30-50 miles of the hospital. If the patient’s home is located far from the hospital, then alternative lodging may be provided. The patient’s caretaker must return patient quickly back to the hospital if health problems surface.
CAR design
CAR-T therapy uses gene therapy to insert a chimeric (synthetic) gene into the genome of a patient’s T cell. This CAR gene will express a CAR protein that localizes into the cell membrane of the T cell. Each CAR-T company designs slightly different CARs.
The extracellular component of this CAR protein is a fragment of an antibody. This antibody fragment (scFv) will specifically bind to one specific target, or antigen. In the case of Yescarta, Kymriah, Tecartus and Breyanzi the antibody fragment binds to CD19. A CD19 CAR will attach to cancerous B cells, which express CD19 on their surface. Unfortunately, healthy B cells also express CD19 on their surface.
The CAR protein contains additional domains that control T cell activity. A linker domain provides flexibility for the antibody fragment to pivot along the T cell surface. A transmembrane domain spans the membrane. Various intracellular domains sit on the inside of the T cell and dictate cellular activity within the T cell. Depending on the CAR-T company’s design, these domains will differ. That said, there is usually a survival domain (CD3-ζ) that initiates downstream activity, including cell survival. The other key intracellular domain is the co-stimulatory domain. The co-stimulatory domain governs the intensity of cytokine production. Cytokines are chemical signals used by immune cells, such as T cells, to help elevate or suppress immune activity.
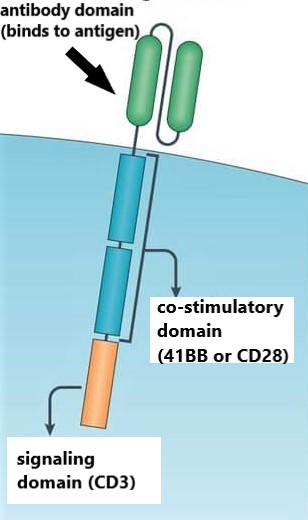
CAR protein situated on T cell membrane
Table below lists key domains for various CAR-T therapies.
| CAR-T company | Therapy | Target | Co-stimulatory domain |
|---|---|---|---|
| Kite/Gilead | Yescarta | CD19 | CD28 |
| Novartis | Kymriah | CD19 | 41BB |
| Juno/BMS | Breyanzi | CD19 | 41BB |
| BMS/Bluebird | Abecma | BCMA | 41BB |
Author’s additional notes: I’ve heard discussion that the 41BB co-stimulatory domain leads to more mild side effects (CRS/neurotoxicity). For patients, this could mean spending less days in a hospital after infusion. That said, there are multiple moving parts in a CAR-T therapy, so it is tricky to assign any outcome to one design element.
Gene therapy component of CAR-T products
Currently, CAR-T cell therapy companies rely on either a lentivirus or a retrovirus to introduce the CAR gene into patient’s T cells. This procedure is called ex vivo gene therapy, because the cells are transfected (genetically engineered) outside of the human body. In the case of commercial CAR-T, the ex vivo gene therapy step occurs at a manufacturing site owned by the CAR-T company. Some hospitals manufacture their own CAR-T cells on site in order to supply cells for clinical trials. In that case, this gene therapy step would occur at the hospital, possibly using Miltenyi Biotec equipment.
Table below lists the type of viral vector used by different CAR-T companies.
| CAR-T company | Therapy | Viral vector |
|---|---|---|
| Kite/Gilead | Yescarta | ϒ-retrovirus |
| Novartis | Kymriah | lentivirus |
| Juno/BMS | Breyanzi | lentivirus |
Additional notes on CAR-T gene therapy:
A lentivirus and a retrovirus are RNA based viruses that can carry a therapeutic gene up to 8 kb in size. After infecting a cell, these RNA based viruses use an enzyme called reverse transcriptase to convert its RNA into DNA. The retrovirus then integrates its viral DNA into the DNA of the host cell (the T cell).
A retrovirus only infect dividing cells. Lentiviral vectors are a type of retrovirus that infects both dividing and non-dividing cells. This is possible because their pre-integration complex (virus shell) can penetrate the intact nucleus membrane of a non-dividing cell.
Both of these viruses integrate randomly into the genome of the T cells. Other viruses used in gene therapy (i.e. AAVs), do not integrate genes into chromosome and instead create non-integrating episomes. The benefit to genome integration is that the therapeutic gene is not diluted out with each cell division.
Efficacy of CAR-T
Both Yescarta and Kymriah are being prescribed for various forms of aggressive lymphoma. A 2019 study showed that about 80% of lymphoma patients receiving CAR-T have a form of relapsed or refractory lymphoma called diffuse large B-cell lymphoma (DLBCL). Chemotherapy has failed to keep these patients in remission. Prior to CAR-T, chemotherapy resistant DLBCL patients did not have many treatment options.
About 60-70% of people with DLBCL have lasting responses to their initial chemotherapy. For people whose DLBCL does not respond (refractory disease) or comes back (relapses) after initial treatment, “the ability to cure [these patients] with conventional therapy is very, very limited,” Dr. Wilson explained.
CAR-T is now commonly prescribed for 3rd line DLBCL patients.
Efficacy data from CAR-T clinical trials
On the table below I include key efficacy metrics from pivotal clinical trials for Yescarta, Kymriah and Breyanzi. Breyanzi is another CD19 CAR-T therapy designed by Juno Therapeutics (now owned by Bristol Meyers Squibb. Breyanzi was approved in 2021.
| CAR-T company | Therapy | Pivotal trial | Overall response rate (%) | Complete response rate (%) |
|---|---|---|---|---|
| Kite/Gilead | Yescarta | Zuma-1 (n=101) | 72 | 51 |
| Novartis | Kymriah | Juliet (n=68) | 50 | 32 |
| Juno/BMS | Breyanzi | Transcend 001 (n=269) | 73 | 53 |
Response reported with 95% exact binomial confidence interval.
Source: ASH 2019 abstracts/FDA labels
Definitions of cancer efficacy endpoints
Objective response rate (ORR)
ORR is the portion of patients with a tumor size reduction of a predefined amount for a defined time period. The FDA has generally defined ORR as the sum of partial responses (PRs) and complete responses (CRs).
Complete response (CR)
CR is defined as the “disappearance of all signs of cancer in response to treatment.” Although CR is preferred over ORR, very few drugs produce high rates of CR (FDA, 2004)
Real world efficacy data, post approval
At the ASH medical conference in December 2019, post approval CAR-T data was presented. This is data collected from treated cancer patients outside of clinical trials. Sometimes efficacy and safety results observed during the clinical trial do not match up to real world data. Fortunately, it appears the response rate (ORR and CR) in real world data is similar to clinical data.
We performed a multicenter retrospective study to include both approved commercial products, axi-cel (yescarta) and tisa-cel (kymriah), given in centers that had the option of prescribing either product. We evaluate patterns of use, efficacy, and safety. (ASH, 2019). Efficacy outcomes in the commercial setting appear similar to responses seen in the pivotal clinical trials.
| CAR-T company | Therapy | Patients infused | Day 30 Overall response rate (%) | Day 30 Complete response rate (%) |
|---|---|---|---|---|
| Kite (Gilead) | Yescarta (axi-cel) | n=149 | 72 | 43 |
| Novartis | Kymriah (tisa-cel) | n=75 | 59 | 44 |
In order to best appreciate the health outcomes of a new therapy, one needs to compare the new therapy results with the standard of care results in the same cancer patient group.
Below, Christopher Manz succinctly describes the efficacy of CAR-T compared to standard of care. In this scenario, standard of care is defined as salvage therapies.
For adults with relapsed or recurrent lymphoma, CAR-T therapies achieve complete responses in 40% to 54% of patients versus 7% of patients treated with other salvage therapies, and more than 30% of CAR-T patients enjoy prolonged disease-free survival.
Safety issues with CAR-T
Cytokine release syndrome
The most common side effect of CAR T-cell therapy is called cytokine release syndrome, or CRS.
CRS is often described as a cytokine storm. Cytokines are chemical signals used by various immune cells to communicate with each other. About 70-90% of patients experience CRS. Fortunately, CRS often passes after five to seven days. Most patients describe CRS as similar to a severe case of the flu, with high fever, fatigue and body aches. It usually starts around the second or third day after infusion. CRS happens because the modified T cells become hyper activated when attacking cancer cells, causing an elevated immune response in the body. Hyper activated T-cells emit cytokine signals to further elevate immune system activity. If these activation signals continue to elevate then CRS can appear.
CRS is commonly treated with corticosteroids or tocilizumab. Tocilizumab can block Interleukin 6 (IL-6) and reverse CRS side effects fairly quickly. 62% of Yescarta patients use tocilizumab for CRS, however only 13% of Kymriah patients require the CRS drug. The CRS difference between these drugs may be attributed to their different co-stimulatory domains (41BB vs. CD28).
Neurotoxicity
CAR-T patients often experience loss of memory or confusion. This neurotoxicity effect is also referred to as CRES. The side effect may arise in the first week after infusion. To monitor CRES, caretakers ask patients a series of basic questions, such as ‘What city are you in?‘. A CRES patient may not know the answer to these questions. Patients eventually recover all of their neurological functions.
The medical community is not entirely clear about the cause for this neurotoxicity effect. It seems that some component of the immune system (i.e. cytokines, T-cells) are reaching the brain after breaching the blood brain barrier, possibly via disruptions in the endothelial membrane. 41% of Yescarta patients experience neurotoxicity; this number is considerably lower for Kymriah patients.
Safety data from CAR-T clinical trials
| CAR-T company | Therapy | Pivotal trial | CRS (%) | Neurotoxicity (%) |
|---|---|---|---|---|
| Kite/Gilead | Yescarta | Zuma-1 (n=101) | 13% | 31% |
| Novartis | Kymriah | Juliet (n=111) | 22% | 12% |
| Juno/BMS | Breyanzi | Transcend 001 (n=269) | 2% | 10% |
CRS and Neurotoxicity data reports % of patients with greater than or equal to grade 3 on intensity scale.
Source: ASH 2019 abstract
Real world safety data, post approval CAR-T
| CAR-T company | Therapy | Patients infused | CRS (%) | Neurotoxicity (%) |
|---|---|---|---|---|
| Kite (Gilead) | Yescarta | n=149 | 13% | 41% |
| Novartis | Kymriah | n=75 | 1% | 3% |
Advantages and disadvantages of CAR-T
Advantages
- CAR-T cell therapy involves a single infusion of modified T cells. An infused patient usually needs to remain inpatient at a hospital for clinical monitoring for about a week. But once that is over, the patient shouldn’t need another treatment. In contrast, a non-Hodgkin lymphoma patient that is undergoing chemotherapy will usually require six months of repeated rounds of chemotherapy.
- CAR-T is a living drug. In theory, its benefits can last as long as the modified T cells remain circulating in the patient’s body. The cancer community is still collecting long term durability data on CAR-T, however one study demonstrates impressive durability. 15 months after CAR-T infusion, 42% of the adult lymphoma patients that received CD19, CAR T-cell therapy were still in remission. This efficacy data is remarkable because these are patients that have already failed to respond to traditional lymphoma treatments.
Disadvantages
- CAR-T is considerably more expensive than chemotherapy. Novartis charges 475K for their Kymriah product (leukemia). Gilead charges 373K for Yescarta. Both private and Medicare based insurance are picking up the tab for these drugs, however, the bigger financial issue is the large amount of hospital charges that accumulate for each treated patient. Oncologists, nurses, clinicians all need to be involved in the CAR-T process. If the patient requires extensive inpatient days post-infusion, then additional hospital costs will accumulate. OHSU in Oregon estimates the hospital will need 1.5M dollars per patient to fully recoup their expenses related to administering CAR-T. Medicare and private insurance companies are less clear about reimbursement for these hospital related costs.
- Cancer cells evolve in our body. Cancer cells want to proliferate and out-compete their surrounding tissue. This often means cancer cells will begin to mask or stop producing a marker such as CD19. B-cells, including lymphoma cells, express this CD19 protein on their surface as CD19 is necessary for proper B cell maturation. CAR-T works by attaching T cells to this CD19 protein. In an attempt to evade destruction, lymphoma cells can become CD19 negative, in which case the CAR-T therapy won’t work. Multiple studies reveal that patients do not respond to CD19/CAR-T if their cancer cells harbor mutated versions of the CD19 protein. Exon skipping events can modify CD19 protein so that cancer cells remain undetected by CAR-T.
- Modifying T cells can pose a manufacturing challenge. Novartis has struggled to deliver modified T cells back to the patient in the same time frame as Gilead. The difference in manufacturing time between these CAR-T companies has been approximately 2-3 weeks vs. 3-5 weeks. Additionally, the FDA has set standards for the health of these manufactured T-cells. Among other criteria, the cells must be more than 80% viable to be delivered as a product to patients. In the real world data presented at ASH 2019, 29 of 102 lymphoma patients treated received out of specification cells, which were below 80% viability. These cells can be offered to patients for free under expanded access protocol. This abstract offers more detail on T cell manufacturing metrics for Kymriah. Novartis found that most of their transfected T cells were central memory T cells of both the helper and killer T cell variety (CD4 and CD8). In general, the analysis concludes that many manufacturing metrics (type of T cells transfected, viability) has little effect on clinical outcomes.
Future directions in CAR-T and other cell therapies
1.) Transition from liquid cancers to solid tumors
The first wave of CAR-T therapies targeted liquid cancers (leukemia lymphoma, multiple myeloma). These are cancers of immune cells (B-cells, plasma cells, etc.) This makes sense as re-engineered T-cells infused back into the patient’s circulatory system will be in direct contact with a cancer cell that circulates in the blood and lymphatic system. Now that we’ve seen proof of concept for liquid cancers, it will be interesting to see what level of success CAR-T companies have targeting tumors outside of the circulatory and lymphatic system. In general, a T-cell can only be used to target a solid tumor if lymphatic vessels and/or T-cells traffic into the region of the body where the tumor exists.
Can a circulating T-cell attack a solid tumor in the brain?
The brain is separated from the rest of the body by a blood–brain barrier (BBB). This BBB is composed of a series of tight cell junctions along brain endothelial cells. This cell layer prevents the vast majority of cancer therapeutic drugs from entering the brain. However, recently we have learned that lymphatic vessels do exist in the brain and we now know T cells can penetrate the BBB and infiltrate the brain. This knowledge opens the door for CAR-T therapies to target glioblastoma tumors. Glioblastoma is an aggressive brain cancer. Clinical trials are now underway that use CAR-T cells directed at a variant of EGFR (epidermal growth factor III). This EGFR protein is expressed on the surface of glioblastoma cancer cells.
Aside from access, solid tumors remain a challenge for additional reasons.
- We need more tumor-cell specific target antigens.
- We must overcome the immuno-suppressive tumor environment.
2.) Reduction in length of hospital stay
Much of the hospital cost associated with CAR-T treatment derives from the number of inpatient days required post-infusion. If the next generation of CAR-T companies can design new treatments with minimal adverse effects, then patients will require fewer days in the hospital after treatment and patient care costs will drop.
This reduction in cost is especially important with patients on Medicare/Medicaid. When the government is paying for the treatment, the hospital often only receives reimbursement for a fraction of the total cost of patient care. If a new CAR-T therapy arrives with a lower rate of adverse events (below 10% of treated patients), then the sting of partial reimbursement becomes much more tolerable for health care providers. And, it goes without saying, that a safer and more tolerable CAR-T therapy will dramatically improve the overall patient experience.
3.) New antigens open up new disease targets
The first wave of CAR-T therapies targeted the CD19 antigen. This strategy has proven fruitful in the realm of lymphoma and leukemia. However, moving forward, the CAR-T community will be targeting other antigens. The table below lists some antigens currently pursued in CAR-T.
| Antigen | Target cells/cancer type |
|---|---|
| CD-19 | B-cells in leukemia/lymphoma (CD19 also presents on healthy B-cells) |
| CD-22 | B-cells in leukemia/lymphoma (more prone to be silenced than CD-19) |
| CD-20 | B-cells in leukemia/lymphoma (may persist on cancerous B-cells after CD-19 loss) |
| BCMA | plasma cells in multiple myeloma |
| CLL-1 | acute myeloid leukemia blasts |
| EGFR | various tumors (i.e. glioblastoma) |
| HER-2 | breast cancer cells |
| WT1 | NSCLC, Mesothelioma (WT1 is an intracellular protein overexpressed in many cancer cells) |
| MUC16 | Ovarian cancer (MUC16 is overexpressed in majority of ovarian cancers) |
| NKG2D | Colorectal cancer, AML, MDS |
| CD30 | Hodgkin lymphoma cells, some non-Hodgkin cells, not commonly found on healthy cells |
| Nectin-4 | expressed on many solid tumor cells, with uniform expression on bladder cancers |
| TF (tissue factor) | overexpressed in many solid tumors, including ovarian, prostate, melanoma and others |
4.) CAR cell therapies beyond T cells
CAR based cell therapy need not be restricted to T-cells. As you would imagine, T-cells will not be the optimal manufactured cell type for every cancer type. Fortunately, scientists can mimic the CAR-T approach by genetically inserting a CAR gene into a variety of cells that circulate in our body.
I will briefly mention two leading T-cell alternatives: NK cells and macrophages.
Natural Killer cells (CAR-NK)
Natural killer cells (NK) can be reprogrammed, in the same manner as T-cells, to create CAR-NK cells.
NK cells are a type of immune cell that can recognize foreign cells without the need for antibodies or the MHC. In theory, CAR-NK offers advantages over CAR-T. CAR-NK don’t undergo rapid clonal expansion and so may not trigger an intense CRS response. CAR-NK don’t require strict MHC matching and so may be more amenable to allogeneic treatments. Additionally, CAR-NK should be able to recognize cancer in the absence of a CAR antigen.
Macrophages (CAR-MA)
Macrophages are another cell type in our immune system. They are part of our non-specific response to foreign pathogens. Macrophages recognize threatening cells and physically engulf the cells, essentially eating the target cell. This process if called phagocytosis.
The benefit to a using a macrophage over a T-cell lies in the macrophage’s ability to thrive in the tumor microenvironment. Tissue inside tumors can be low on oxygen and nutrients. Macrophages perform better in this sub-optimal environment and, therefore, are thought to penetrate tumors deeper than T-cells. Scientists out of UPenn formed a company named Carisma and are leading the charge with CAR-MA. In 2018, AbbVie and others financed Carisma with 53M in Series A funding.
5.) Shift from personalized cell therapy to allogeneic CAR-T
Many of the logistical challenges associated with CAR-T administration could be alleviated with allogeneic CAR-T, or off the shelf cell therapy. Yescarta and Kymriah are both considered autologous CAR-T because the patient’s own T cells are modified and turned into the product. In contrast, allogeneic, or off the shelf, CAR-T cells are collected from healthy donors, re-engineered, frozen down, then applied to many different patients as needed. In theory, one set of allogeneic CAR-T cells could be produced for each cancer type. Each cancer center could store these cryopreserved cells in frozen IV bags. Cancer patients would arrive at the hospital and receive an infusion with the appropriate IV bag of T-cells. In this sense, the allogenic cell therapy is simply pulled off the shelf and administered.
If this allogeneic strategy is successful, then much of the manufacturing supply chain required to create each personalized CAR-T treatment could be eliminated. Allogeneic CAR-T could introduce a massive cost reduction into the CAR-T treatment landscape.
A central challenge for allogeneic CAR-T companies lies in the ability to silence immune rejection, also known as graft vs. host disease. If T-cells from another human are pumped into a patient’s blood, the recipient’s immune system may recognize those cells as foreign and attack them.
Allogeneic CAR-T companies must develop the capacity to silence immune rejection. For example, Celyad, one of the first companies to begin human clinical trials with allogenic CAR-T, is silencing the TCR molecules on the surface of T-cells with a peptide called TCR Inhibiting Molecule. This could prevent the patient’s immune system from recognizing the cells as foreign.
Other allogeneic companies, such as Allogene, are using gene editing to eliminate the T cell receptor gene (TCR). Allogene also plans to remove the CD52 gene from allogeneic CAR-T cells. This CD-52 alteration allows an immune suppressing anti-CD52 treatment to be delivered in conjunction with CAR-T, without the risk of reducing modified T cell activity.
Allogenic cells can also be HLA matched in an attempt to decrease immune rejection. Our T-cells naturally express various forms of HLA complexes. Think of this like the different blood types (A, B, AB, O). There are about six common versions of HLA alleles expressed on T-cells. An allogeneic therapy could ensure that the recipient receives an appropriate, HLA matched batch of T-cells.
If proper steps are taken to decrease immune rejection and if the lympho-depleting chemotherapy step is optimized, then the durability of an allogeneic treatment should increase.
List of Allogeneic CAR-T companies*
| Organization | CAR-T product | Target antigen/disease | Development stage |
|---|---|---|---|
| Allogene | ALLO-501 | CD19/NHL lymphoma | Phase I |
| UCART19 | CD19/NHL lymphoma | Phase I | |
| ALLO-715 | BCMA/Multiple Myeloma | Phase I | |
| Cellectis | UCART-123 | CD123/ AML | Phase I |
| Celyad | CYAD-101, using Natural Killer cells | NKG2D/Colorectal cancer | Phase I |
| Chinese People’s General Hospital | UCART019 | CD19/lymphoma/leukemia | Phase 1/2 |
| Mesothelin CAR-T | mesothelin/solid tumors | Phase 1 | |
| CRISPR | CTX 110 | CD19/NHL | Phase 1 |
| CTX 120 | BCMA, Multiple Myeloma | Phase 1 | |
| CTX 130 | CD70, solid tumors | Phase 1 | |
| Precision Bioscience/Servier | PBCAR-0191 | CD19/NHL, ALL | Phase 1 |
| Shanghai Bioray Lab | CD19 UCART | CD19/NHL, ALL | Phase 1 |
Allogeneic companies listed are all in clinical development. Preclinical companies are excluded.
If I’m missing an organization, please reach out.
References
Bagley, Stephen J., et al. “CAR T-cell therapy for glioblastoma: recent clinical advances and future challenges.” Neuro-oncology 20.11 (2018): 1429-1438.
Bradley D Hunter, Caron A Jacobson, CAR T-Cell Associated Neurotoxicity: Mechanisms, Clinicopathologic Correlates, and Future Directions, JNCI: Journal of the National Cancer Institute, Volume 111, Issue 7, July 2019, Pages 646–654
Depil, S., et al. “‘Off-the-shelf’allogeneic CAR T cells: development and challenges.” Nature Reviews Drug Discovery (2020): 1-15.
Klingemann, Hans. “Are natural killer cells superior CAR drivers?.” Oncoimmunology 3.4 (2014): e28147.
Kogan, Allan Jay, and Melinda Haren. “Translating cancer trial endpoints into the language of managed care.” Biotechnology healthcare 5.1 (2008): 22.
Minutolo, Nicholas G., Erin E. Hollander, and Daniel J. Powell Jr. “The Emergence of Universal Immune Receptor T Cell Therapy for Cancer.” Frontiers in oncology 9 (2019).
Otero, Dennis C., and Robert C. Rickert. “CD19 function in early and late B cell development. II. CD19 facilitates the pro-B/pre-B transition.” The Journal of Immunology 171.11 (2003): 5921-5930.
Schläger, Christian, et al. “Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid: allogeneic car-t companies” Nature 530.7590 (2016): 349.
Sotillo, Elena, et al. “Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy.” Cancer discovery 5.12 (2015): 1282-1295.